Pipeline
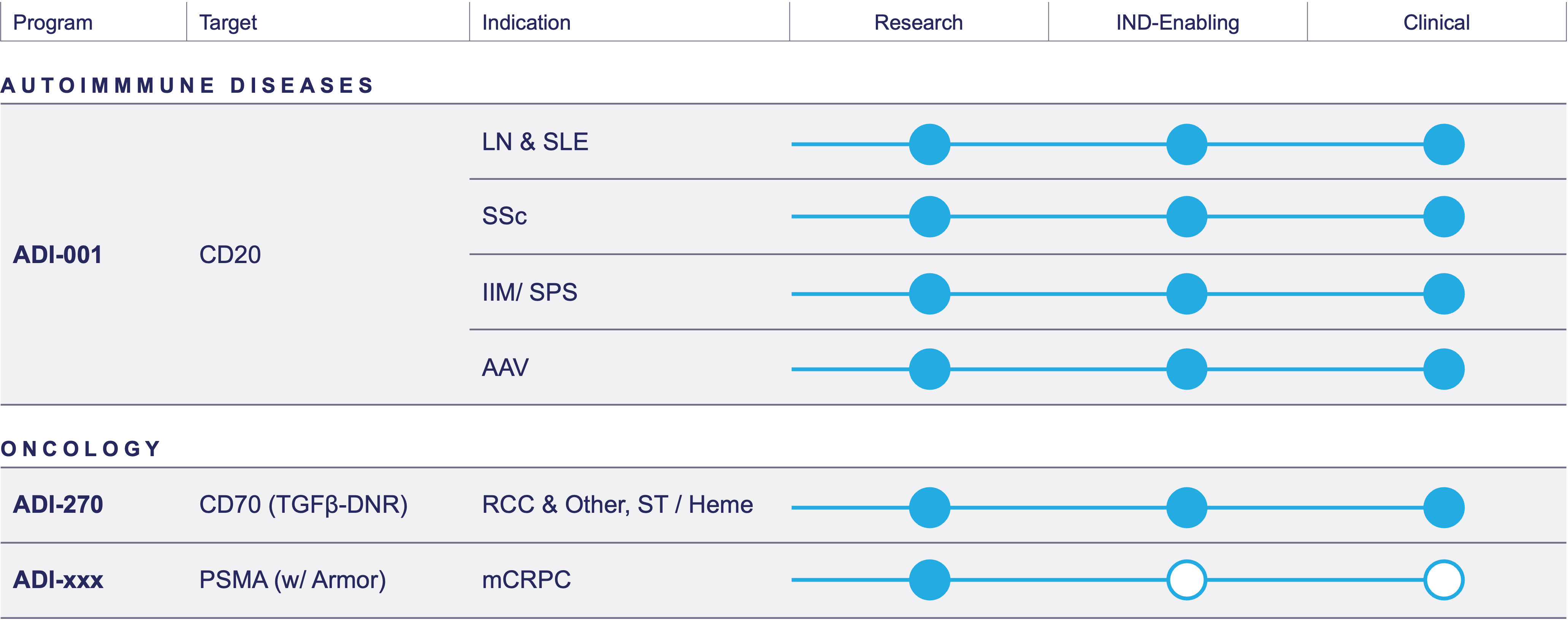
AAV= anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis; ccRCC= Clear cell renal cell carcinoma; IIM= idiopathic inflammatory myopathy; IND= Investigational new drug; LN= lupus nephritis; mCRPC= Metastatic castration-resistant prostate cancer; PSMA= Prostate specific membrane antigen; SLE= systemic lupus erythematosus; SPS= stiff person syndrome; SSC= systemic sclerosis; ST= Solid tumor
Adicet Bio is a clinical stage biotechnology company discovering and developing allogeneic gamma delta T cell therapies for autoimmune diseases and cancer.
Adicet is advancing a pipeline of “off-the-shelf” gamma delta T cells, engineered with chimeric antigen receptors (CARs), to facilitate durable activity in patients.
ADI-001
Our lead product candidate, ADI-001, is a first-in-class investigational allogeneic gamma delta CAR T cell therapy currently being developed in autoimmune indications. It’s being studied in a Phase 1 study in lupus nephritis (LN), systemic lupus erythematosus (SLE), systemic sclerosis (SSc), idiopathic inflammatory myopathy (IIM), stiff person syndrome (SPS) and anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (AAV).
In Autoimmune Diseases
ADI-001 is ideally suited for the treatment of autoimmune diseases given its robust B-cell depletion consistent with other autologous CAR T therapies tested in autoimmune diseases, its tissue homing, and the safety data observed in our oncology Phase 1 study.
In June 2024, the FDA granted ADI-001 Fast Track Designation for the potential treatment of relapsed/refractory class III or class IV LN.
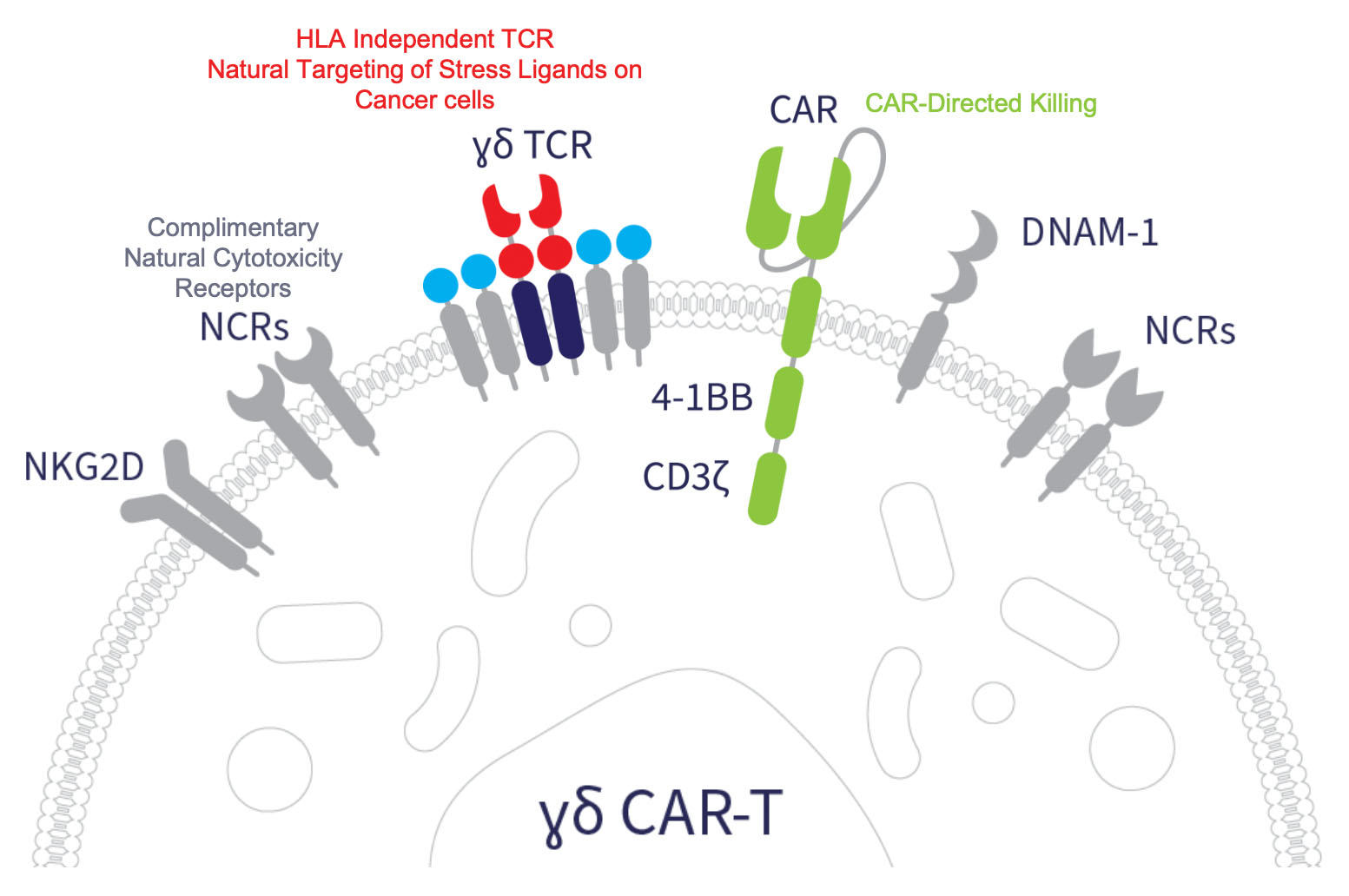
ADI-270
- ADI-270, an armored CD70 CAR γδ T cell targeting CD70 via the CD27-ligand, has shown superiority to scFv (single-chain variable fragment) CARs1
- Innate and adaptive targeting mechanisms associated with activity in solid tumors such as renal cell carcinoma (RCC) and other indications
- Armoring via dominant negative receptor; addresses TGFβ in Tumor Microenvironment2
- Lead CAR demonstrated potency and improved serial killing, and resilience against suppressive factors and graft vs host rejection
- Homing and activity of gamma delta 1 T cells demonstrated in RCC
-
Received IND clearance and FDA Fast Track Designation
1 Sauer et al. Blood (2021)
2 Junker et al. Cytokine (2000)
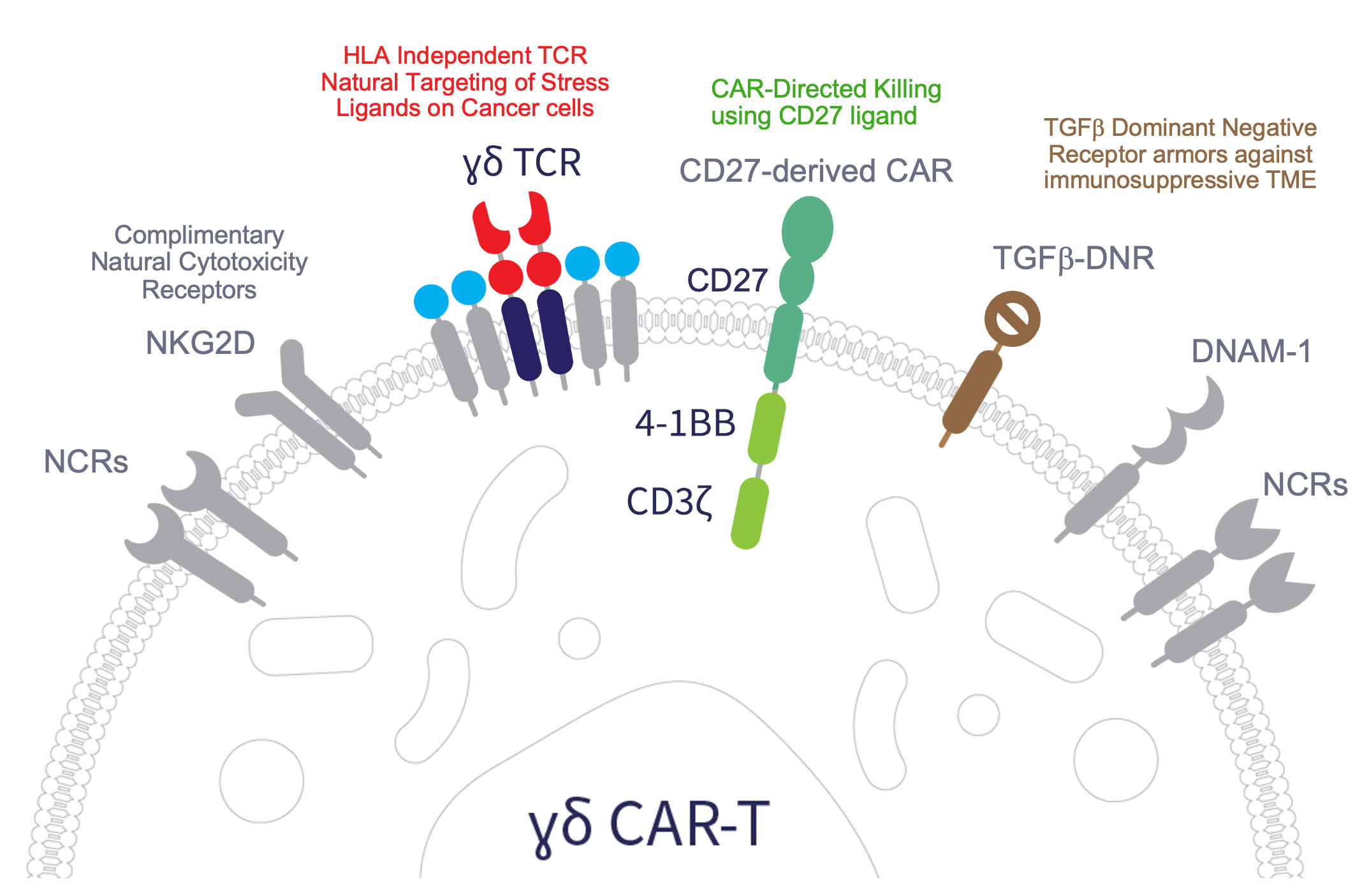
Armored PSMA CAR Gamma Delta T Cell Program
- Potent CAR construct active against heterogeneous PSMA
- Three mechanisms of action designed to address tumor heterogeneity
- Homing of gamma delta 1 T cells documented in mCRPC
- Inclusion of armoring to address suppressive TME
- No significant CRS and ICANS demonstrated with Adicet CAR gamma delta 1 T cells in clinical trials reported to-date
Liu et al. Cancer Res. (1997)
Expanded Access Policy
Expanded Access refers to the use of an investigational therapy outside of a clinical trial for potential treatment of a serious or life-threatening condition. Adicet is a clinical stage biotechnology company discovering and developing allogeneic gamma delta T cell therapies for autoimmune diseases and cancer. Consistent with our commitment to bring innovative, safe, and effective therapies to patients, we are focused on conducting the clinical trials necessary to gain regulatory approvals to make our therapies available to patients as quickly as possible. Participation in one of our clinical trials is the best way to access Adicet gamma delta T cell investigational therapies. As such, Adicet does not provide access to our investigational therapies on an Expanded Access basis at this time.
We encourage patients interested in our investigational therapies to learn more about our ongoing studies by visiting clinicaltrials.gov. Treating physicians may request additional information about Adicet’s Expanded Access policy by contacting inquiries@adicetbio.com
Presentations and Publications
ADI-270, an Armored Allogeneic Anti-CD70 CAR γδ T Cell Therapy, Demonstrates Robust CAR-Directed and -Independent Anti-Tumor Activity Against Hematologic and Solid Tumor Models Compared to Conventional CAR αβ T Cells
ASGCT Oral Presentation | 2025
ADI-001: An allogeneic CD20-targeted γδ CAR T cell therapy with potential for improved tissue homing in autoimmune indications
ACR24 Oral Session Scientific Presentation | 2024
ADI-270: An Armored Allogeneic Anti-CD70 CAR γδ T cell Therapy Candidate Designed for Multiple Solid and Hematological Cancer Indications
ASGCT Oral Presentation | 2024
ADI-270: An Armored Allogeneic Anti-CD70 CAR γδ T cell Therapy Designed for Multiple Solid and Hematological Cancer Indications
ASGCT Abstract | 2024
ADI-925: an allogeneic off-the-shelf Chimeric Adapter (CAd) γδ T cell therapy targeting NKG2D ligand-expressing cancers
SITC Poster | 2023
Disruption of the cytokine signaling checkpoint CIS enhances serial-killing and anti-tumor activity of CAR-engineered γδ T cells
SITC Poster | 2023
Characterization of Allogeneic CAR γδ1 T Cell Therapy for Prostate Cancer Targeting a Novel Dimeric Epitope on PSMA (Prostate-Specific Membrane Antigen)
EORTC-NCI-AACR Poster | 2023
ADI-270: an armored allogeneic “off-the-shelf” CAR γδ T cell therapy targeting CD70+ cancers
ASGCT Poster | 2023
Preclinical Discovery And Evaluation of Allogeneic “off-the-shelf” γδ CAR T Cells Targeting B7-H6+ Tumors
SITC Poster | 2022
Allogeneic “off-the-shelf” γδ T cells modified with CD27-containing CAR for targeting CD70+ cancers
SITC Poster | 2022
Innate-Enhanced Chimeric Adaptors (CAd): A Newly-Described Approach for Augmenting Potency of γδ T Cell Immunotherapy
SITC Poster | 2022
Preclinical Discovery and Characterization of Allogeneic Anti-PSMA γδ CAR-T Therapy for Prostate Cancer
SITC Poster | 2022
A Phase 1 Study of ADI-001: Anti-CD20 CAR-Engineered Allogeneic Gamma Delta1 (γδ) T Cells in Adults with B-Cell Malignancies
ASH Abstract | 2022
A Phase 1 Study of ADI-001: Anti-CD20 CAR-engineered Allogeneic Gamma Delta (γδ) T cells in Adults with B Cell Malignancies
ASCO Abstract | 2022
Evaluation of non-gene edited allogeneic “off-the-shelf” Vδ1 γδ CAR T cells targeting CD20 for B cell malignancies
ISCT Poster | 2022
Allogeneic CD20-targeted γδ T cells exhibit innate and adaptive antitumor activities in preclinical B-cell lymphoma models
Clinical & Translational Immunology (CTI) | 2022
Gammaretroviral and lentiviral vector manufacture: brief overview
Cell and Gene Therapy Insights | 2021
Effects of IL-2 and IL-15 on the proliferative and antitumor capacities of allogeneic anti-CD20 CAR-engineered γδ T cells in a 3D B cell lymphoma spheroid assay
SITC Poster | Spheroids 10 video | 2020
A Novel γδT Cell Product Targeting CD20 for the Treatment of B Cell Malignancies
ASGCT Poster | 2019
Leveraging Bioprocess Platform Technology for the Development of a Robust, Scalable, and Economic Manufacturing Process of Allogeneic CAR-T Cell Therapy Products
ECI Poster | 2019
T-cell Receptor-like Antibodies Directed Against Intracellular Tumor Targets for Immunotherapy of Solid Tumors
PEGS Boston Poster | 2019

